
BRPES-Series Bio-Burden Reduction Grade Polyethersulfone
BRPES-Series High Purity Bio-Burden Reduction Grade Filter Polyethersulfone Cartridges are validated and 100% integrity tested; providing bio-burden and small particle removal across a wide range of food & beverage, biological liquids, and intermediate bulk pharmaceutical fluids.

BRPES-Series Overview
BRPES-Series High Purity Bio-Burden Reduction Grade Filter Polyethersulfone Cartridges are validated and 100% integrity tested; providing bio-burden and small particle removal across a wide range of food & beverage, biological liquids, and intermediate bulk pharmaceutical fluids. The BRPES-Series is constructed using a unique single-layer hydrophilic asymmetric polyethersulfone membrane. This construction offers broad chemical compatibility, high flow-rates at low pressure drops, and low extractables. BRPES cartridges are ideal as either a final filtration stage or as an extremely effective prefilter to a sterilizing stage. Manufactured in a clean-room environment to maintain high standards of purity and cleanliness.
Typical Applications
- Cell Culture Media
- Large Volume Parenterals (LVP’s)
- Pharmaceutical Bulk Chemical Solutions
- Diagnostics
- Blood and Serum Fractions
- Purified Water
- Beer, Wine & Spirits
- Juice & Soft Drinks
- Bottled Water
Construction Materials
- Membrane: Polyethersulfone
- Support Media: Polypropylene
- End Caps: Polypropylene
- Center Core: Polypropylene
- Outer Support Cage: Polypropylene
- O-Rings/Gaskets: Buna, EPDM, Silicone, Teflon® Encapsulated Viton®, Viton®, Teflon® Encapsulated Silicone
Operating Conditions
- Change-Out ΔP (recommended): 35 PSID
- Temperature (max): 176˚F (80˚C)
- Differential Pressure (max): 72 PSID (3.4 bar) at 68˚F (20˚C)
Sterilization
- Hot Water: 85-95˚C for 30 min., max dP 7 PSI
- Steam Sterilization: 134˚C for 30 min., max dP 7 psi; 100 cycles
Toxicity
All polypropylene components meet the specifications for biological safety per USP Class VI – 121 ̊C for plastics.
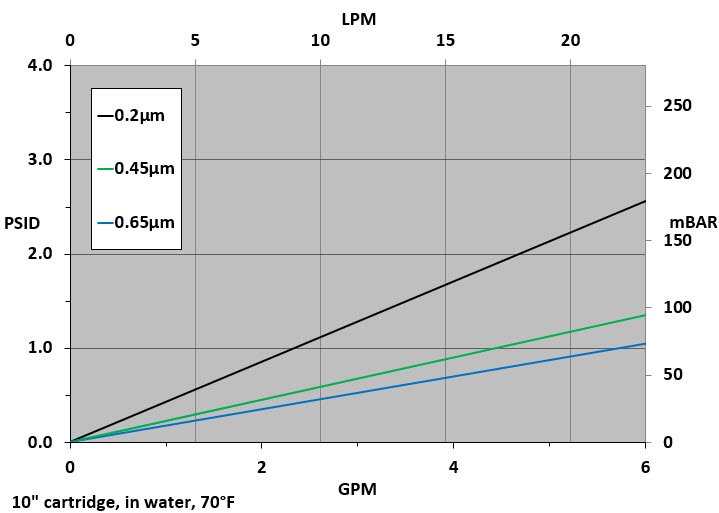
Flow Rate vs Pressure Drop
Dimensions
- Length: 10 to 40 inches ( 254 to 101.6 cm) nominal
- Outside Diameter: 2.70 inches (7.0 cm) nominal
Food Safety Compliance
Materials of construction comply with FDA regulations for food and beverage contact use as detailed in the US Code of Federal Regulations, 21CFR. Materials used to produce filter media and hardware are deemed safe for use in contact with foodstuffs in accordance with EU Directives 1935/2004, and/or 10/2011.
BRPES-Series Ordering Specifications
| Rating (μ) | 0.2, 0.45, 0.65 |
| Length | 10″, 20″, 30″, 40″ |
| End Cap Style | 2 = DOE Flat Gasket 3 = 222 w/ Fin 4 = 22 w/ Flat Cap 6 = 226 w/ Flat Cap 7 = 226 w/ Fin 28 = 222 3-Tabs w/ Fin |
| O-Rings/Gaskets | B = Buna-N E = EPDM S = Silicone T = Teflon® Encapsulated Viton® V = Viton® Z = Teflon® Encapsulated Silicone |
Microbial Retention Performance
| Rating | Challenge Microbe | Log Reduction Value |
| 0.2μ | Brevundimonas diminuta | >8.0 |
| 0.45μ | Lactobacillus lindneri, Serratia marcescens | >8.0 |
| 0.65μ | Lactobacillus lindneri, Saccharomyces cerevisiae | >8.0 |







