
PPES-Series Pharmaceutical Grade Polyethersulfone
PPES-Series High Purity Pharmaceutical Grade Polyethersulfone Filter Cartridges are ideal for sterile filtration and pharmaceutical and biological solutions clarification.

PPES-Series Overview
PPES-Series High Purity Pharmaceutical Grade Polyethersulfone Filter Cartridges are ideal for sterile filtration and pharmaceutical and biological solutions clarification. Each PPES cartridge is integrity tested during manufacturing and is supported by a validation guide for regulatory compliance. Low protein binding, the broad chemical compatibility characteristics of the polyethersulfone membrane, and exceptional flow rate vs pressure drop make the PPES-Series the ideal choice for various valuable and/or critical pharmaceutical solutions. PPES cartridges are fully validated as sterilizing grade filters in accordance with HIMA and ASTM F838-05 guidelines.
Typical Applications
- Vaccines
- Large Volume Parenteral (LVP’s)
- Water For Injection (WFI)
- Diagnostics
- Ophthalmics
- Cell and Tissue Culture Media
- Protein Solutions
- Serum and Blood Products
Construction Materials
- Membrane: Polyethersulfone
- Support Media: Polypropylene
- End Caps: Polypropylene
- Center Core: Polypropylene
- Outer Support Cage: Polypropylene
- O-Rings/Gaskets: Buna, EPDM, Silicone, Teflon® Encapsulated Viton®, Viton®, Teflon® Encapsulated Silicone
Operating Conditions
- Change-Out ΔP (recommended): 35 PSID
- Temperature (max): 176˚F (80˚C)
- Differential Pressure (max): 72 PSID (5.0 bar) at 68˚F (20˚C)
Sterilization
- Hot Water: 85˚C – 95˚C, 30 min., max. dP 7 psi
- In-Line Steaming: 134˚C, 30 min., max. dP 7 psi; 100 cycles
Toxicity
All polypropylene components meet the specifications for biological safety per USP Class VI – 121 ̊C for plastics.
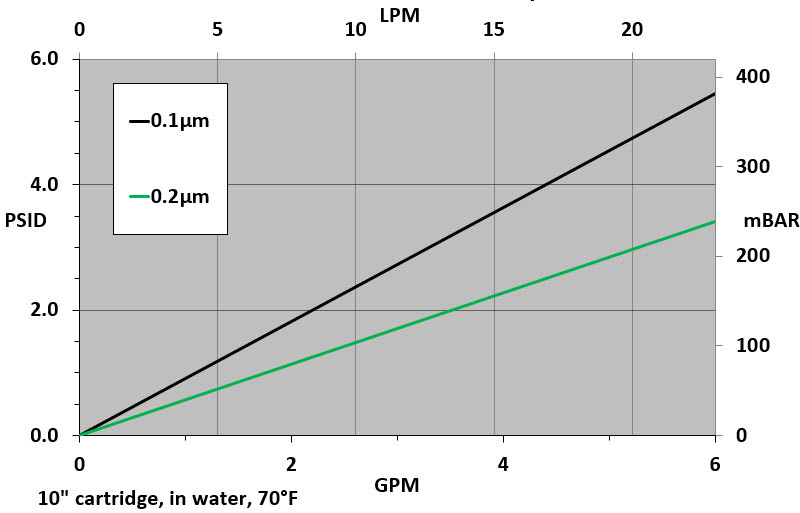
Flow Rate vs Pressure Drop
Dimensions
- Length: 10 to 40 Inches (25.4 to 101.6 cm) nominal
- Outside Diameter: 2.78 inches (7.06 cm) nominal
Food Safety Compliance
Materials of construction comply with FDA regulations for food and beverage contact use as detailed in the US Code of Federal Regulations, 21CFR. Materials used to produce filter media and hardware are deemed safe for use in contact with foodstuffs in accordance with EU Directives 2002/72/EC, 1935/2004, and/or 10/2011.
PPES-Series Ordering Specifications
| Micron Rating(μ) | 0.1, 0.2 |
| Length | 10″, 20″, 30″, 40″ |
| End Cap Styles | 2 = DOE Flat Gasket 3 = 222 w/ Fin 4 = 222 w/ Flat Cap 6 = 226 w/ Flat Cap 7 = 226 w/ Fin 28 = 222 3-Tabs w/ Fin |
| O-Rings/Gaskets | B = Buna E = EPDM S = Silicone T = Teflon® Encapsulated Viton® V = Viton® Z = Teflon® Encapsulated Viton |
Microbial Retention Rating
| Rating | Challenge Microbe | Log Reduction Value |
| 0.1 | Acholeplasma laidlawii (Mycoplasma) | >7 |
| 0.2 | Brevundimonas diminuta | >7 |







